MoNo – das Unternehmen
 Die Firma MoNo chem-pharm Produkte GmbH wurde im Jahr 2000 als Herstellungsbetrieb gegründet und hat seine Wurzeln im Traditionsunternehmen Sigmapharm Arzneimittel GmbH, welches seit mehr als siebzig Jahren im Dienste der Gesundheit steht. Jahrzehntelanges Know-how, modernste Produktionsanlagen auf dem Stand der Technik und Lohnfertigung auf höchstem Niveau: MoNo chem-pharm Produkte GmbH versteht sich als universeller Dienstleister und Hersteller. Mehr zum Unternehmen
Die Firma MoNo chem-pharm Produkte GmbH wurde im Jahr 2000 als Herstellungsbetrieb gegründet und hat seine Wurzeln im Traditionsunternehmen Sigmapharm Arzneimittel GmbH, welches seit mehr als siebzig Jahren im Dienste der Gesundheit steht. Jahrzehntelanges Know-how, modernste Produktionsanlagen auf dem Stand der Technik und Lohnfertigung auf höchstem Niveau: MoNo chem-pharm Produkte GmbH versteht sich als universeller Dienstleister und Hersteller. Mehr zum Unternehmen
Herstellung
Die Firma Mono chem-pharm Produkte GmbH hat sich auf die Entwicklung und Produktion aller Arten von pharmazeutischen Liquida (steril und nicht steril, wässrig, ölig, alkoholisch, viskos) spezialisiert. Das Herzstück unserer Firma sind modernste Anlagen zur aseptischen Herstellung steriler flüssiger Produkte. Sämtliche Herstellungsprozesse werden gemäß aktueller GMP-Richtlinien und ISO-Normen strengstens kontrolliert und überwacht, sodass wir höchste Qualität garantieren können. Rund um die Herstellung bieten wir als Team von MoNo ein komplettes Leistungsspektrum für Produktentwicklung, Prüfmusterherstellung, Prozessvalidierungen und Analytik an. Unsere Zusammenarbeit mit der Sigmapharm Arzneimittel GmbH erweitert unser Dienstleistungsspektrum im Bereich der Erstellung von Einreichdokumentationen, bei der Entwicklung von Packmitteldesign, sowie bei Lagerhaltung und Versandlogistik.
Karriere
Weil wir Großes erreichen wollen, brauchen wir Mitarbeiterinnen und Mitarbeiter mit Visionen, die unsere Wertvorstellungen…
Sterile Lösungen
STERILE LÖSUNGEN UNSTERILE LÖSUNGEN KLEINCHARGEN-SERVICE STUDIEN-MEDIKATION SERIALISIERUNG UMVERPACKUNG Unsere Produktionsstandorte in Wien und Hornstein sind…
Unsterile Lösungen
STERILE LÖSUNGEN UNSTERILE LÖSUNGEN KLEINCHARGEN-SERVICE STUDIEN-MEDIKATION SERIALISIERUNG UMVERPACKUNG Neben unserer Kernkompetenz der aseptischen Herstellung und…
Kleinchargenservice
STERILE LÖSUNGEN UNSTERILE LÖSUNGEN KLEINCHARGEN-SERVICE STUDIEN-MEDIKATION SERIALISIERUNG UMVERPACKUNG Nicht immer werden Arzneimittel oder Medizinprodukte in…
Studienmedikation
STERILE LÖSUNGEN UNSTERILE LÖSUNGEN KLEINCHARGEN-SERVICE STUDIENMEDIKATION SERIALISIERUNG UMVERPACKUNG Das Clinical Trial Service von MoNo unterstützt…
Serialisierung
STERILE LÖSUNGEN UNSTERILE LÖSUNGEN KLEINCHARGEN-SERVICE STUDIEN-MEDIKATION SERIALISIERUNG UMVERPACKUNG Wir sind für die Arzneimittelfälschungsrichtlinie gerüstet: In…
Produktentwicklung
 Neben der Lohnproduktion flüssiger steriler und nicht steriler Produkte bieten wir zusätzlich die komplette Bandbreite an Services zur Produktentwicklung an. Wir unterstützen Sie mit unserer Expertise und entwickeln gemeinsam Ihr neues Produkt von der Idee bis hin zur Produktion und Markteinführung. Mehr zur Produktentwicklung
Neben der Lohnproduktion flüssiger steriler und nicht steriler Produkte bieten wir zusätzlich die komplette Bandbreite an Services zur Produktentwicklung an. Wir unterstützen Sie mit unserer Expertise und entwickeln gemeinsam Ihr neues Produkt von der Idee bis hin zur Produktion und Markteinführung. Mehr zur Produktentwicklung
Qualitätskontrolle
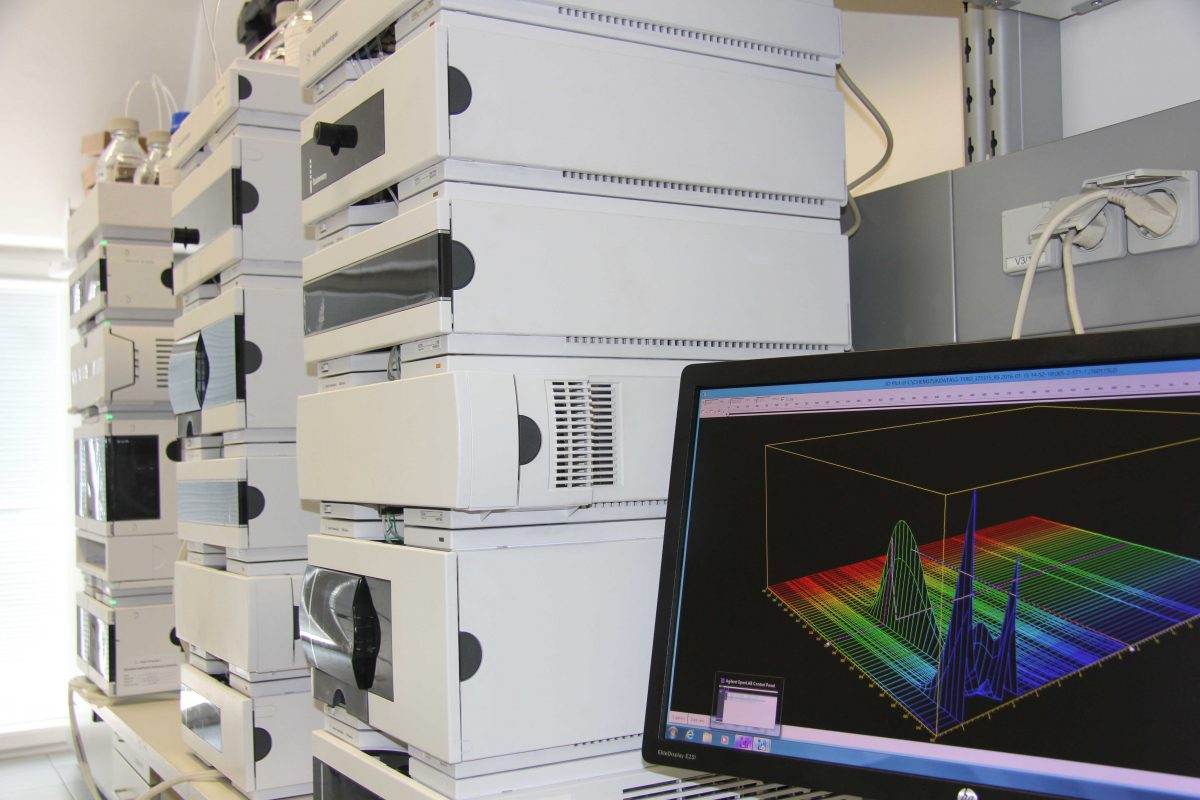 Unser hochqualifiziertes und erfahrenes QC-Team führt chemische und mikrobiologische Analysen mittels state-of-the-art Technik durch. Wir prüfen Regularien-konform vom Rohstoff über Packmittel bis hin zum Fertigprodukt und reagieren flexibel auf Ihre individuellen Bedürfnisse. Mehr zur Qualitätskontrolle
Unser hochqualifiziertes und erfahrenes QC-Team führt chemische und mikrobiologische Analysen mittels state-of-the-art Technik durch. Wir prüfen Regularien-konform vom Rohstoff über Packmittel bis hin zum Fertigprodukt und reagieren flexibel auf Ihre individuellen Bedürfnisse. Mehr zur Qualitätskontrolle
©2019 Mono.co.at | Datenschutzerklärung | Haftungsausschluss | Impressum